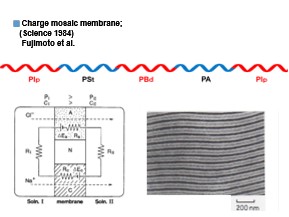
Charge mosaic membrane is made of Poly(Isoprene-block-Styrene-block-Butadiene-block-Amine-block-Isoprene) synthesized by anionic polymerization, the polyamine moiety is then quaternization, and the polydiene moiety is cross-linked. Finally, the polystyrene moiety was sulfonated. Figure 1 shows a schematic diagram of the tetrablock polymer, a transmission electron microscope (TEM), and a diagram of the principle of ion transmission, showing that the cation exchange layer and anion exchange layer, which are separated from each other by an insulating layer, are arranged in regular alternation. The principle of ion transmission is described in detail in MEMBRANE, 8(4), 212-224 1983 and MEMBRANE, 16(4), 233-238 1991 (Miyaki et. al.).
The liquid crystal polymer in Figure 2 is made by coupling a small molecule liquid crystal to Poly(p-hydroxystyrene), the central chain of Poly(Styrene-b–p-Hydroxystyrene-b-Styrene). It can be used as a reversible recording medium because fine dots can be formed or disappeared at any position by irradiation of laser light. Incidentally, the coupling rate of small molecule liquid crystals is 100%.
This method covers the disadvantage of anionic polymerization that the number of polymerizable monomers is small. We have synthesized DDS, oxygen-enriched membranes, hemicellulose blockpolymers, peptide polymers, water filtration membranes, etc. The coupling method is one of the most important synthetic methods because it can easily create functional polymers by simply changing materials.
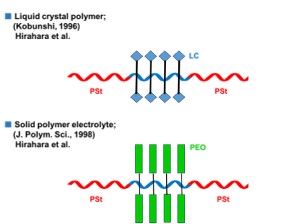
Solid polymer electrolyte was obtained by grafting ethylene oxide from PHSt, the central chain of P(St-b-HSt-b-St), by the growth method. We call this polymer a block-graft copolymer (TGE). Despite the total molecular weight reaching 40 x 104, the PDI (GPC) = 1.08 was very sharp.
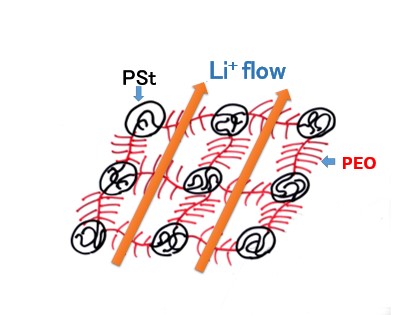
Figure 3 shows a schematic diagram of a solid polymer electrolyte, with PSt as the sphere and PEO (polyethylene oxide) as the matrix, where PSt and PEO chains are responsible for the mechanical strength and ionic conductivity of the membrane, respectively.
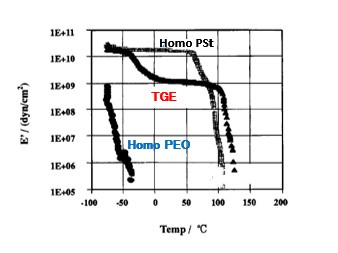
Homopolymer of PSt exhibits high E’ from -60°C to +70°C, but decreases rapidly from around Tg (glass transition temperature). Low molecular weight PEO is liquid at room temperature, so there is no E’. On the other hand, TGE exhibits a high mechanical strength of 1E+9 up to around +110°C, even though the PEO chains in the liquid state form a matrix. Block copolymers have two Tg, and TGE also has a PEO Tg around -40°C and a PSt Tg around +110°C observed. This is one of the reasons for the high ionic conductivity of this tough film. (Figure 4) Furthermore, when TGE was impregnated with an electrolyte to produce a semi solid polymer electrolyte, the ionic conductivity was increased more than 1000 times without much loss of film strength.
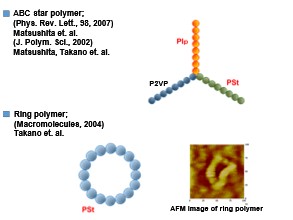
Matsushita et al. realized quasicrystalline structures in polymers by self-assembling ternary star polymers composed of Polystyrene, Polyisoprene, and Poly(2-vinylpyridine). [Figure 5]
Takano et al., synthesized and characterized in detail a ring polymer of PSt with a cyclization ratio as high as 95%. The AFM image was taken over 25 years ago, so the image is not very clear, but the shape of the ring polymer could still be clearly seen.
