8-1. Synthesis and Characterization of PSt-b-PMMA
Polystyrene-block-polymethyl methacrylate (PSt-b-PMMA) has been extensively studied because of the similar surface energies of the PSt and the PMMA blocks, which leads to the perpendicular orientation upon simple thermal annealing without neutral layer. However, it was reported that the resolution limit was 13 nm half pitch (hp) and a lower dry etch selectivity (2:1).
To achieve sub-10 nm feature size, we synthesized PS-b-PMMA with the narrow molecular weight distribution (Mw/Mn < 1.1), and the composition PS = 0.5 by the living anionic polymerization technique. The domain spacing (D) of alternative lamellar structure of the obtained sample with molecular weight of 25k was determined to be 20.5 nm (hp = D/2 = 10.3 nm) by SAXS measurement (Figure 1-a). It was confirmed that the hp is smaller than the resolution limit of PSt-PMMA predicted previously1.
Figure 17 shows the polymerization scheme of P(St-b-MMA). polymerization of P(St-b-MMA) itself is simple, but it is difficult to monodisperse MMA, a polar monomer, and the PDI was only 1.04 even using the breakable method.
Table 1. Morphological observation and quantitative analysis of domain size.
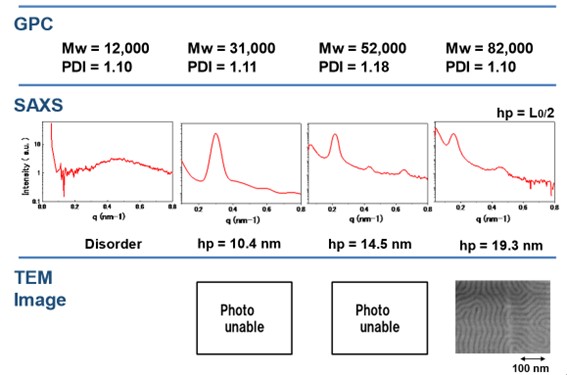
Table 1 shows the Mw, PDI, SAXS, and TEM results for P(St-b-MMA). Here, SAXS was measured using a Spring 8 (Riken) GI-SAX; TEM was obtained by staining samples with OsO4 or RuO4. Although the PDI is not so good since this data is about 10 years old, SAXS and TEM observations still confirmed the formation of a clear microphase-separated structure.
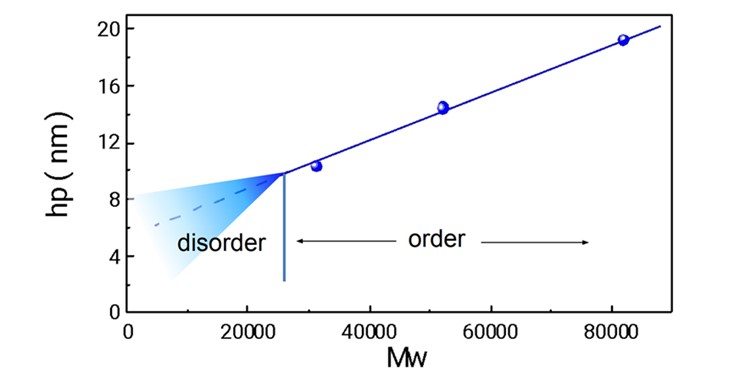
Figure 18 plots hp versus Mw in Table 1, showing a clean linearity between Mw and hp. On the other hand, it was found that PSt and PMMA compatibilizer with each other around hp of 10 nm, forming a microphase-separated structure down to a smaller region than the theoretical calculation results.
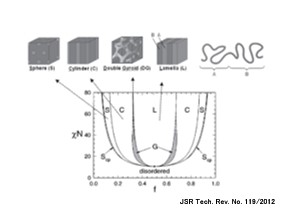
Diblock copolymers, in which two mutually incompatible polymer components are covalently linked, form a microphase separation by intermolecular assembly of the same polymers. In this process, the interfacial curvature at the time of assembly changes according to the molecular chain length ratio f between the two polymer components. As a result, regular morphologies such as spherical, cylinder, gyroid, and lamellar structures are formed (Figure 19). These regular structures can be applied to semiconductor fine patterning. On the other hand, there is a limit to how much the molecular weight can be lowered, and eventually the material will become miscible; for P(St-b-MMA), this limit is around 10 nm.
Directed Self-Assembly (DSA) of block copolymers (BCPs) is one of the candidates for the next generation technique for semiconductor patterning at the length scale in sub10 nm regime. Polystyrene-block-polymethyl methacrylate (PSt-PMMA) has been extensively studied because of the similar surface energies of the PSt and the PMMA blocks, which leads to the perpendicular orientation upon simple thermal annealing without neutral layer. However, it was reported that the resolution limit was 13 nm half pitch (hp) and a lower dry etch selectivity (2:1). To achieve sub-10 nm feature size, we synthesized PS-b-PMMA with the narrow molecular weight distribution (Mw/Mn < 1.1), and the composition PS = 0.5 by the living anionic polymerization technique. The domain spacing (D) of alternative lamellar structure of the obtained sample with molecular weight of 25k was determined to be 20.5 nm (hp = D/2 = 10.3 nm) by SAXS measurement (Table 1). It was confirmed that the hp is smaller than the resolution limit of PSt-PMMA predicted previously1.
8-2. New concept polymer: random-block copolymers
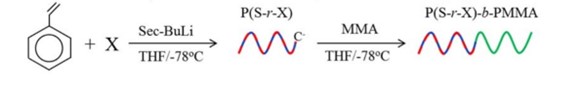
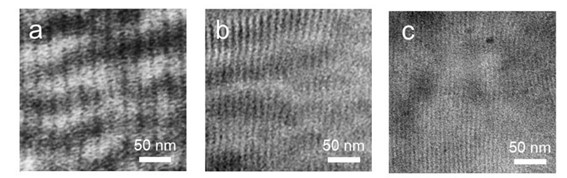
We synthesized a series of poly(styrene-random-X)-block-polymethyl methacrylate P(S-r-X)-b-PMMA where X means Silicone containing monomer. One of the P(S-r-X)-b-PMMA sample showed narrow domain spacing of 20.0 nm (10.0 nm hp) by SAXS measurement (Figure 20).
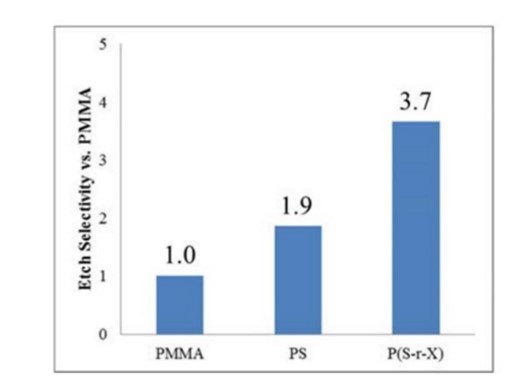
For the evaluation of the dry etch selectivity, PMMA, PS and also P(St-r-X) with various compositions were synthesized. As a result, the etch selectivity of P(S-r-X) with a certain composition was estimated to be over three times higher than that for PMMA (Figure 24).
Investigation of the BCPs with narrower half pitch and a higher dry etch selectivity is still going on.
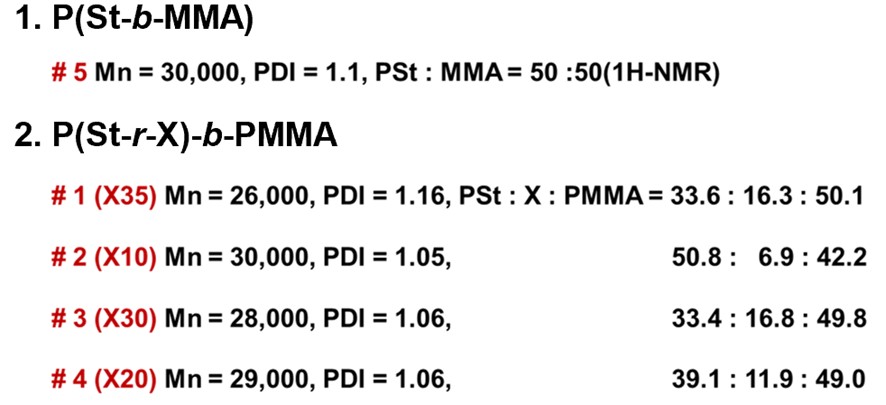
Table 2. Molecular weight, PDI (GPC) and composition ratio of P(St-r-X)-b-PMMA
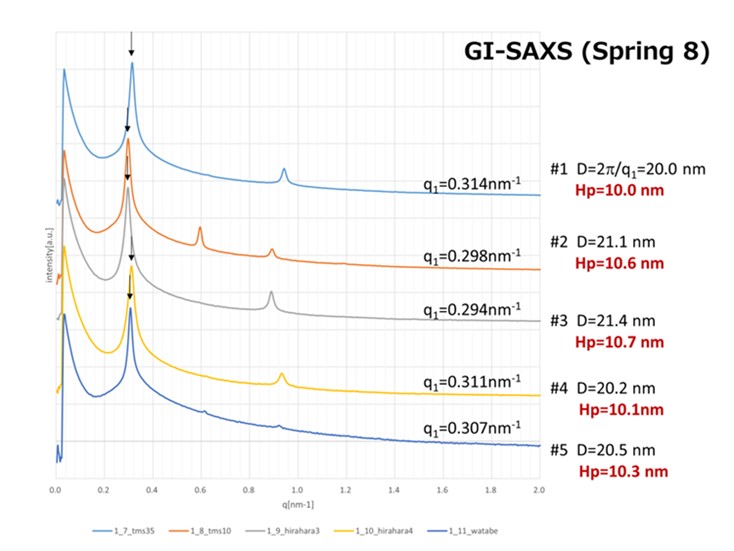
Investigation of the BCPs with narrower half pitch and a higher dry etch selectivity is still going on.
8-3. AB diblock copolymer and ABAB tetrablock copolymer
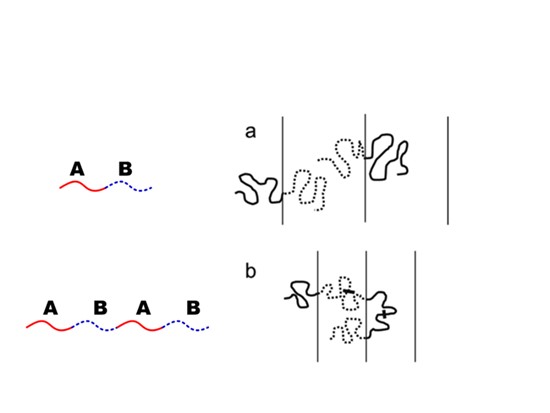
To prepare the narrower domain of microphase-separated structure by block copolymers, utilization of multiblock copolymer effective. In the case of AB diblock copolymer, the diblock copolymers have only end blocks that always take tail conformation, while multiblock copolymers such as ABAB have a middle block that can take either a loop and bridge conformation. Comparing between the chain dimension of a tail conformation and that of loop/bridge conformation with the same molecular weight, the latter should be smaller than the former. Accordingly, the domain spacing of multiblock copolymer with many block number should be shorter than that for simple diblock copolymer as shown in Figure 24.
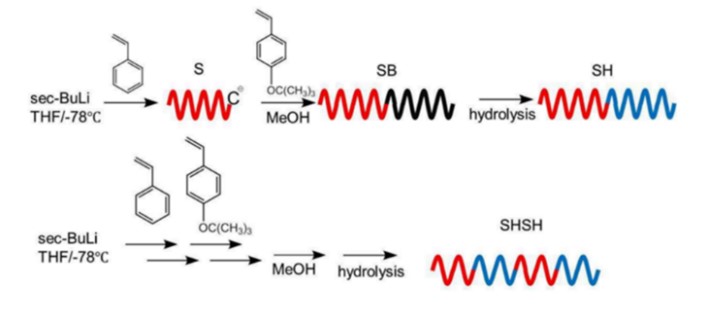
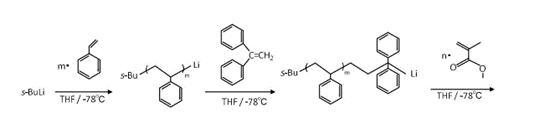
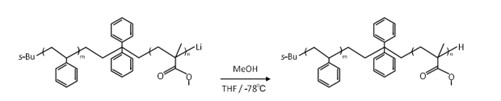
Block copolymers were synthesized by living anionic polymerization by multi-step sequential monomer addition in tetrahydrofuran with sec-BuLi as initiator at -78℃ under vacuum or argon atmosphere. After being quenched with methanol, the obtained polymer was precipitated in excess amount of methanol, and freeze-dried. Poly(4-tert-butoxystyrene) (B) was transformed into Poly(4-hydroxystyrene) by hydrolysis with hydrochloric acid in solvent. Example of the synthetic for SH diblock and SHSH tetrablock copolymer are shown in Scheme 1.
Table 3. Morphological observation and quantitative analysis of domain size.
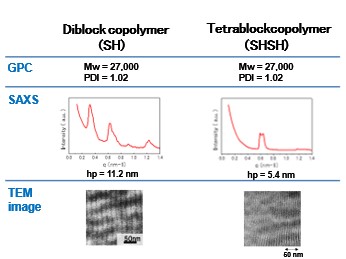
8-4.Scontaining diblock copolymers and tetrablock copolymers
Table 4. Quantitative analysis of domain size. (diblock copolymers)
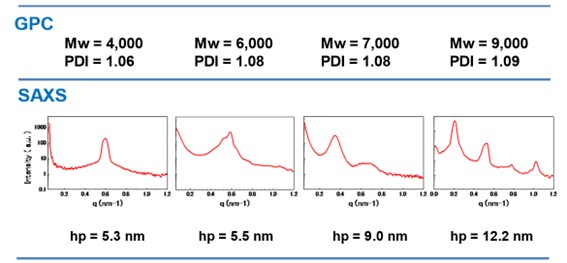
Table 5. Quantitave analysis of domain size. (tetrablock copolymers)
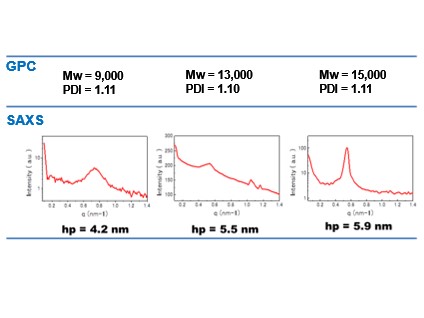

Figure 25 compares the hp to Mw of diblock and tetrablock copolymers. The copolymers are the same High-chi polymers. Since the tetrablock slope is looser than the diblock slope, the different in hp is smaller even if the molecular weights of the polymer deviate slightly. Also, at the same molecular weight, the tetrablock exhibits a smaller hp. Which is an advantageous method for making micro-phase domains. (hp of less than 4nm may be achievable)
I have heard that devices manufactured using ASML’s TWINSCAN NXE: 3400B (NA 0.33, wavelength 13.5 nm) have L/S and Ch of 15-25 nm. The latest High-NA 0.55 is said to have a wavelength of 8 nm, but even so, it would be difficult to get L/S below 10 nm. (Figure 26)
Furthermore, when it comes to High-NA 0.75, we do not know when the equipment will be ready. Our High-chi DSA reached hp 4.2 nm more than 8 years ago, and Sub 4 nm will be achieved soon. On the other hand, the words “3 nm generation” and “2 nm generation” are being bandied about without any clear evidence, but the actual L/S and Ch sizes are far from this. The DSA we have synthesized is an actual value measured by SEM, GI-SAXS, TEM, etc., so it is a reliable value. What generation should we call the hp = 4.2 nm that we have already achieved? I think it has reached a level that we can call 0.5 nm generation or 0.1 nm generation, but what do you think?
